We specialize in
Designing solutions for Cells and Tissue Banks, medical entities, cosmetic and pharmaceutical companies;
Preparation of documentation for Cells and Tissue Banks for inspection in the light of the government licensing requirements;
Implementation and maintenance of international standards for tissue and cell banks;
Implementation of guidelines for the transport of biological material (both domestic and international, including the IATA guidelines for biological materials Category B - potentially infectious);

Advanced Therapy Medicinal Product
We will help you to establish cell or tissue bank in Poland according …

Decontamination kit
Skin/personal items decontamination kit IPP-95 for Toxic Warfare …

Tissue collection kits
BMCT Kits for tissue collection, storage and transportation are …
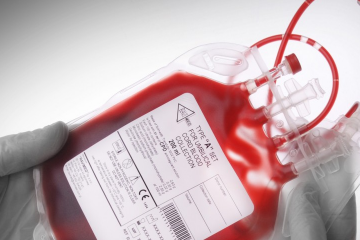
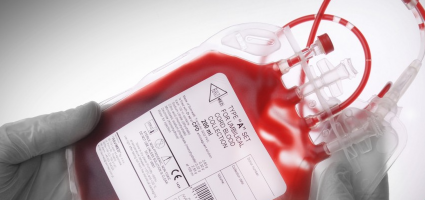
Blood collection systems
A relationship goes through numerous phases during its life cycle, and it is obvious for it to have a few rough patches. However, the strength of a relationship is reflected in how the couples deal with those ...

Decontamination kit
Skin/personal items decontamination kit IPP-95 for Toxic Warfare Agents is designed for individual skin protection and decontamination in case of contamination with Toxic Warfare Agents (TWA)...
Testimonials
We thank BMCT for essential support, so that we can enjoy our success
Public Stem Cell Bank RCNTwww.rcnt.pl/en/